Liquid Biopsy Assays
Liquid biopsy has revolutionized cancer care and is now widely used for cancer screening, diagnosis, and response to targeted therapies. GTL has established a wide collection of liquid biopsy assays using plasma cell free DNA (cfDNA) to detect circulating tumor DNA (ctDNA).
- cfDNA/ctDNA extraction and QC.
- cfDNA/ctDNA tumor mutation detection and CNV detection.
- cfDNA/ctDNA fragmentomics analysis.
- cfDNA/ctDNA methylation analysis.
A liquid biopsy is a minimally invasive medical test that involves sampling and analyzing non-solid biological tissue, primarily blood. Unlike traditional biopsies that require the surgical removal of tissue samples, liquid biopsies can provide a snapshot of a patient’s health status through a simple blood draw. Here’s an overview of key aspects:
Applications
- Cancer Detection and Monitoring:
- Early Detection: Liquid biopsies can identify cancer-related biomarkers before tumors are detectable via imaging.
- Treatment Monitoring: They help track the effectiveness of treatments by measuring changes in biomarker levels.
- Detection of Recurrence: Regular monitoring can identify cancer recurrence earlier than traditional methods.
- Genetic Testing:
- Circulating Tumor DNA (ctDNA): Detects fragments of DNA shed by tumors into the bloodstream, providing genetic information about the cancer.
- Circulating Tumor Cells (CTCs): Identifies intact cancer cells that have broken away from the primary tumor.
- Non-Cancer Applications:
- Prenatal Testing: Non-invasive prenatal testing (NIPT) analyzes fetal DNA in the mother’s blood to screen for genetic abnormalities.
- Transplantation: Monitoring for organ transplant rejection through donor-derived cell-free DNA (dd-cfDNA).
Techniques and Biomarkers
- Circulating Tumor DNA (ctDNA):
- Fragments of DNA released into the bloodstream by cancer cells. Analysis of ctDNA can reveal mutations, copy number variations, and methylation changes.
- Circulating Tumor Cells (CTCs):
- Intact cancer cells that are shed from the primary tumor into the bloodstream. These can be isolated and analyzed to understand the biology of the tumor.
- Exosomes:
- Small vesicles released by cells containing proteins, RNA, and DNA. Exosomes can provide information about the state of the cells from which they originate.
Advantages
- Non-Invasive: Reduces the need for surgical procedures, minimizing risk and discomfort.
- Real-Time Monitoring: Allows for continuous monitoring of disease progression and treatment response.
- Early Detection: Can detect diseases at an earlier stage, potentially improving outcomes.
Challenges
- Sensitivity and Specificity: Detecting low levels of biomarkers in the blood can be challenging.
- Standardization: Lack of standardized protocols and guidelines for sample collection, processing, and analysis.
- Interpretation of Results: Requires advanced bioinformatics tools and expertise to interpret complex data.
PacBio Onso Sequencer
PacBio Onso sequencer. We are actively working to improve the detection sensitivity and accuracy with newer sequencing technologies. The newest instrument is PacBio Onso ultra-accurate short read platform.
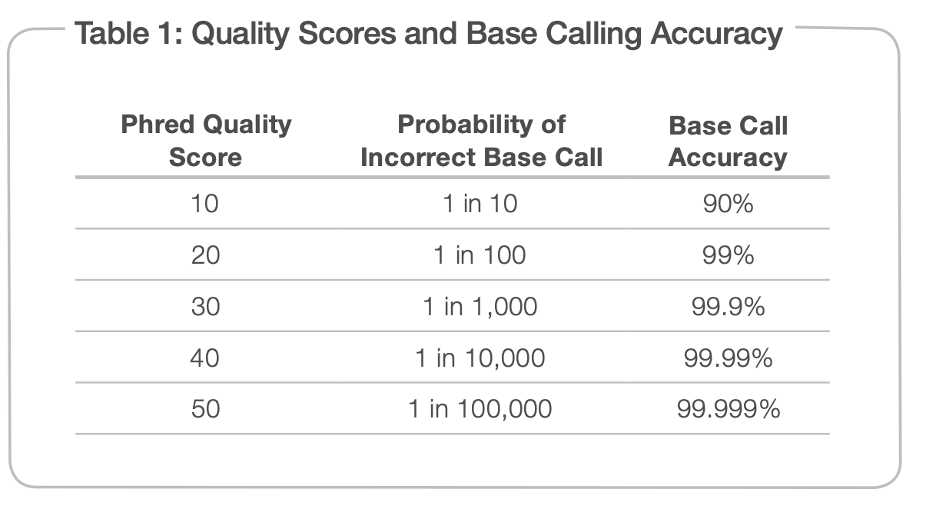
This platform uses sequencing by binding (SBB) chemistry which is different than the sequencing by synthesis (SBS) chemistry used by Illumina platforms. On Illumina platforms, Q30 is the general accepted quality score for sequencing. This means the probability of incorrect base call is 1/1000. The SBB on PacBio Onso can achieve Q40 or Q50 for most of the reads. This means the probability of incorrect base call is 1/10000 or 1/100000 (see table) and the sequencing reads are much more accurate than regular Illumina sequencing. This makes PacBio Onso system much better for cfDNA/ctDNA applications, where most tumor mutations are at very low frequencies and often below the noise level on Illumina platform. The PacBio Onso system enables us to detect tumor mutations at < 1% or <0.1% level.